FDA Warns about Counterfeit Botox Products
Incidents from use by licensed practitioners and unlicensed individuals
Real Botox Packaging

Real Botox Cosmetic Packaging

Real Botox Packaging
Counterfeit Botox Products
The U.S. Food and Drug Administration (FDA) has issued a critical alert concerning the presence of counterfeit Botox products in various states, posing severe risks to consumers.
These unauthorized products have been used for cosmetic purposes and have led to multiple adverse events, including hospitalizations.
Symptoms reported are serious and mimic those caused by botulinum toxin spreading within the body, such as blurred vision, difficulty swallowing, and muscular weakness.
Counterfeit incidents
The counterfeit incidents have occurred both through licensed practitioners and unlicensed individuals, often in informal settings. These fake products may originate from unlicensed sources and can be contaminated, ineffective, or unsafe.
The FDA is actively collaborating with the Centers for Disease Control and Prevention (CDC), state health departments, and AbbVie—the legitimate manufacturer of Botox—to trace, investigate, and eliminate these counterfeit products from the market.
Guidelines for health care professionals
Health care professionals are urged to ensure that any Botox products are purchased from authorized sources as mandated by federal law. They should look for signs of counterfeiting before using the products on patients, thereby avoiding potential risks.
Advice for Consumers
Consumers should seek immediate medical attention if they experience any symptoms post-injection that could indicate the administration of counterfeit Botox.
It is crucial for consumers to verify that their health care provider is using authentic Botox from an approved source and that the provider is properly licensed.
Identifying Genuine Botox Products
Genuine Botox products are available in specific dosages and packaging details, exclusively manufactured by AbbVie. The FDA-approved Botox and Botox Cosmetic are labeled with “OnabotulinumtoxinA” and are packaged in 50-, 100-, and 200-unit vials.
Any product that deviates from these specifications, such as differing dosage units or non-English packaging, should be considered suspect.
Report Counterfeit Botox
Both consumers and health care providers are encouraged to report any suspected counterfeit Botox products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program. enhancing the efforts to safeguard public health.
Adverse reactions should also be reported to help the FDA monitor and respond to the ongoing situation.
Conclusion
The presence of counterfeit Botox is a significant threat to public health. Vigilance in sourcing and administering Botox is crucial for safety. Always confirm the authenticity of the product and the credentials of the provider before proceeding with any treatment.
Links
Read the FDA’s drug safety notification.
Report suspected counterfeit Botox products here.
Read about the potential dangers of counterfeit products.
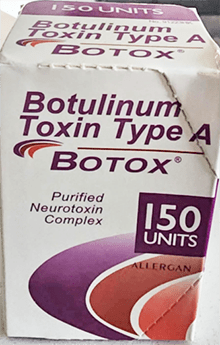
Counterfeit Botox Packaging
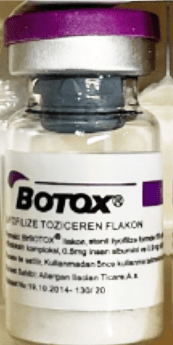
Counterfeit Botox Vial
