FDA Warning: Epinephrine Nasal Solutions
Threat of packaging and labeling mixups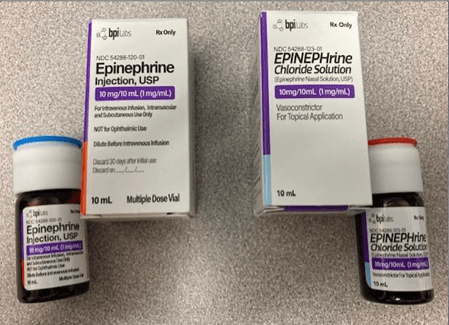
Left: BPI Labs’ FDA-approved Epinephrine Injection with blue lid on the bottle.
Right: BPI Labs’ unapproved EPINEPHrine Chloride Nasal Solution with red lid on the bottle.
Courtesy: FDA.
A Potentially Dangerous Mix-Up
The U.S. Food and Drug Administration (FDA) has issued a critical warning to health care professionals regarding the use of unapproved epinephrine nasal solutions manufactured by BPI Labs LLC and Endo USA. The agency is urging medical providers to avoid these products due to serious risks associated with potential mix-ups between nasal and injectable epinephrine formulations.
The Risk of Confusion
Epinephrine is a life-saving medication used in emergency situations to treat severe allergic reactions, anaphylaxis, and cardiac arrest. However, not all epinephrine products are created equal. While injectable epinephrine is approved by the FDA and required to be sterile for intravenous administration, nasal epinephrine solutions do not meet the same sterility requirements and should never be injected.
Unfortunately, the nasal and injectable products from BPI Labs and Endo USA share similar packaging and labeling, making them difficult to distinguish from one another. This similarity has led to instances where health care professionals mistakenly injected the nasal solution instead of the approved sterile injectable formulation. Since nasal solutions are not sterile, injecting them into a patient can result in severe infections, which can be life-threatening, particularly in vulnerable individuals.
Recalls and FDA Actions
The issue of product confusion is not new. Since 2016, the FDA has received over 25 reports of health care professionals mistakenly using unapproved epinephrine nasal solutions in place of injectable epinephrine. Most recently, in 2024, a patient was reportedly injected with the nasal solution instead of the proper intravenous medication.
Recognizing the severity of this issue, Endo USA voluntarily recalled its unapproved Adrenalin Chloride Solution (Epinephrine Nasal Solution, USP) on December 20, 2024. The FDA had also advised BPI Labs to recall its unapproved EPINEPHrine Nasal Solution as early as December 12, 2024. Despite multiple follow-ups from the FDA, BPI Labs has yet to take action, leaving its product on the market and increasing the risk of continued medication errors.
What Health Care Providers Need to Know
Given the ongoing risk, the FDA is urging all health care professionals to take the following precautions:
- Do not use unapproved epinephrine nasal solutions from BPI Labs and Endo USA.
- Double-check labels and packaging to ensure that the correct, FDA-approved injectable epinephrine is being administered.
- Report any adverse events or medication errors involving epinephrine products to the FDA’s MedWatch program.
Final Thoughts
The FDA’s warning highlights the critical importance of medication safety in clinical settings. While Endo USA has taken responsible action by recalling its unapproved nasal epinephrine solution, BPI Labs has yet to follow suit, leaving a potentially dangerous product in circulation. Health care professionals must remain vigilant in distinguishing between nasal and injectable epinephrine products to prevent harmful medication errors.
For the latest updates and more information, visit the FDA’s official website or report concerns through the MedWatch Adverse Event Reporting Program.
