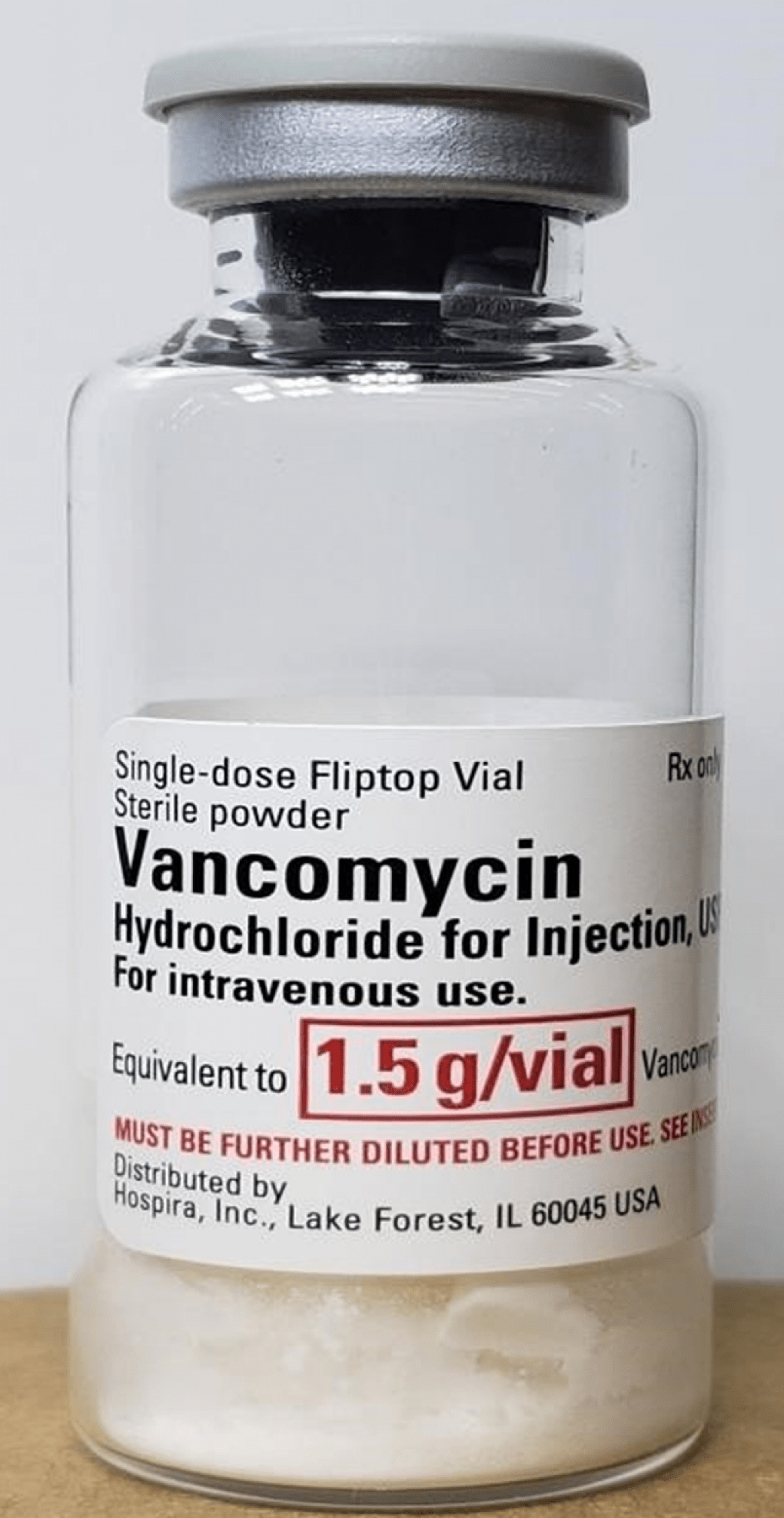
Hospira, Inc., a division of Pfizer, announced a Class I recall of its Vancomycin Injection product due to visible glass particles that were identified in one vial of the drug. The recall involves lot 33045BA of Vancomycin Hydrochloride Injection USP 1.5g single dose flip top vial with an expiration date of 01 September 2023.
About the drug
Vancomycin Hydrochloride is an antibiotic indicated to treat strains of methicillin-resistant staphylococci, and has also shown to be effective in treating bone, skin, and lower respiratory tract infections. It is also used to treat patients who are allergic to penicillin, those who do not respond to other antimicrobials, and for infections cause by antimicrobial resistant vancomycin-susceptible organisms.
Potential adverse events
If ingested intravenously, glass particles may cause local irritation and swelling, antigenic or allergic reactions, vasculitis/ phlebitis, and microvascular blockage, to include pulmonary embolism. If the drug is taken orally, glass particles may cause gastrointestinal trauma. At the time of the recall announcement, Pfizer had not received any reports of adverse events associated with the issue.
Product distribution and actions
The product lot was distributed from June 23 to September 19 of this year, to wholesalers, hospitals, and other institutions throughout the United States and Puerto Rico. Anyone in possession of the drug should stop distribution, discontinue use, and immediately quarantine any products bearing that lot number.
Communication
The FDA Recall Announcement has information for how to report an adverse event related to Vancomycin Hydrochloride, and contact information for Pfizer drug safety and medical information sources.