Medos International Recalls CEREBASE DA Guide Sheath
Due to cracking of its distal catheter shaftUrgent Class I Recall Notice
Medos International Sàrl has issued an urgent recall for the Cerenovus CEREBASE DA Guide Sheath, a neurovascular catheter commonly used in procedures requiring precise navigation and access to blood vessels in the brain. The recall was initiated due to reported fractures of the distal catheter shaft, posing serious risks during surgical procedures.
Reason for Recall
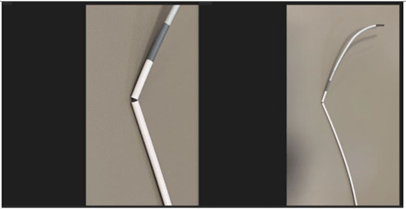
The recall was prompted by complaints of fractures in the distal catheter shaft of the CEREBASE DA Guide Sheath. These fractures could lead to surgical procedural delays, vascular injury, hemorrhage, and in rare cases, embolism. Three injuries have been reported, emphasizing the urgency of the recall.
Affected Parties
Healthcare professionals who utilize the CEREBASE DA Guide Sheath in neurovascular procedures are directly impacted by this recall. Additionally, patients undergoing procedures requiring precise access to brain blood vessels may be affected.
Actions Required
Affected customers have been urged to take immediate action:
- Examine inventory and quarantine affected products.
- Communicate the recall to relevant personnel.
- Arrange for the return of affected products to the originating facility.
- Complete and return the Business Reply Form (BRF) to Johnson & Johnson MedTech.
- Contact Medos for further instructions and to arrange product returns.
Product Details
The recalled products include the Cerenovus CEREBASE DA Guide Sheath, identified by part numbers GS9070SD, GS9080SD, GS9090SD, and GS9095SD, with product code QJP. The manufacturing dates range from July 7, 2023, onwards, and distribution occurred between June 14, 2023, and December 14, 2023. A total of 1,343 devices have been recalled in the United States.
Conclusion
The safety of patients and healthcare professionals is paramount, necessitating swift action in response to this recall. Healthcare facilities and individuals impacted by the recall should follow the outlined steps to ensure the prompt return and replacement of affected CEREBASE DA Guide Sheath devices. For further inquiries or assistance, customers can contact Medos International at OneMD-Field-Actions@its.jnj.com.
Links
Read the FDA recall notice here.
