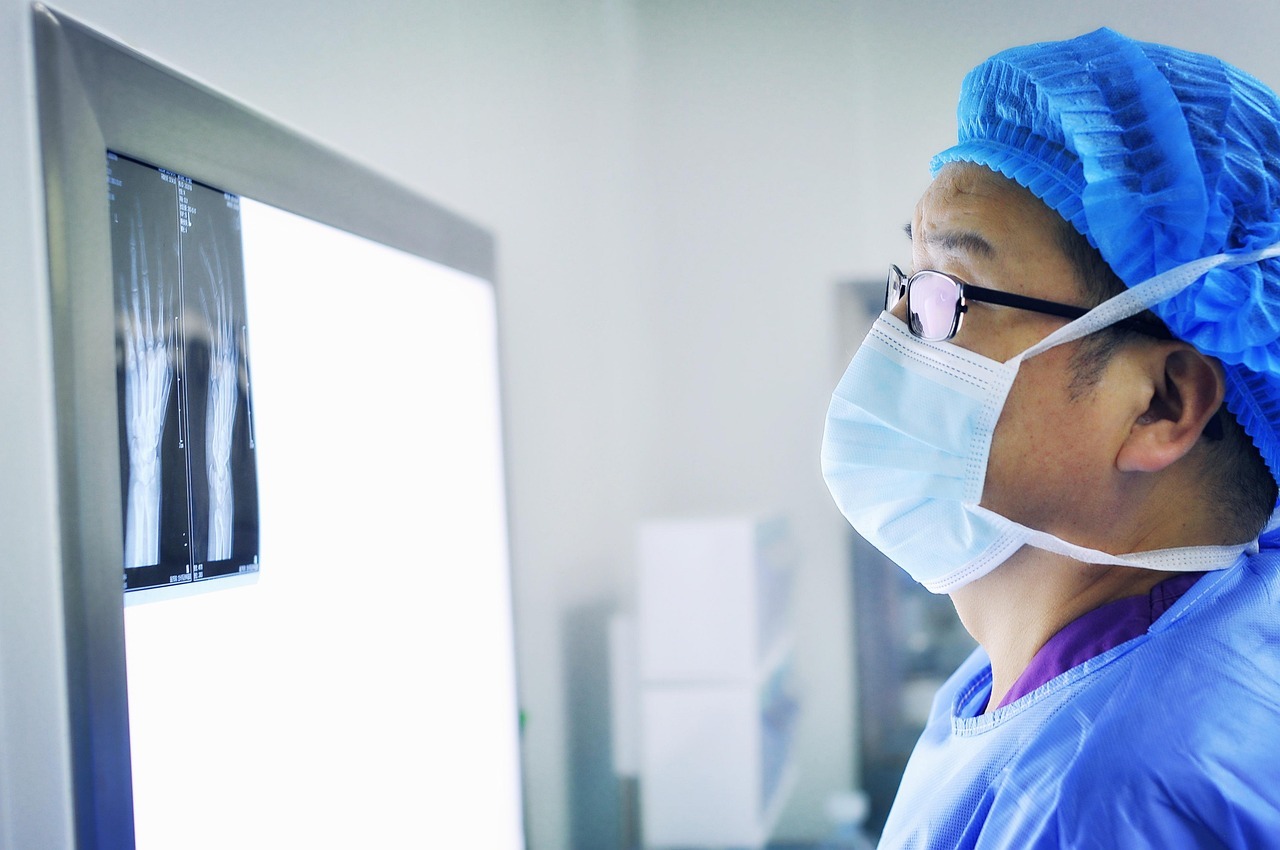
Introduction & Historical Context
Implantable orthopedic devices, such as hip and knee replacements, spinal fixation hardware, and bone screws or plates, have revolutionized modern medicine by restoring mobility and reducing pain for millions of patients worldwide. These devices are designed to integrate with the skeletal system and support or replace damaged bones, joints, or connective tissues.
The history of orthopedic implants dates back over a century. Early versions were made of ivory or glass, but rapid developments in metallurgy and biomaterials during the 20th century brought about the use of stainless steel, cobalt-chrome alloys, and later, titanium and medical-grade polymers. By the 1960s, total hip and knee replacements had entered widespread clinical use.
Fun Fact: The Charnley hip prosthesis, introduced in the 1960s, is considered the prototype for modern total hip arthroplasty.
Scientific & clinical accomplishments
Orthopedic implants are among the most successful surgical interventions in modern healthcare, offering life-changing outcomes for patients suffering from osteoarthritis and rheumatoid arthritis, traumatic injuries or fractures, congenital deformities, and bone tumors or necrosis.
Technological innovations
For modern-day implantable orthopedic devices include:
- Wear-resistant ceramic-on-ceramic bearings
- 3D-printed, patient-specific implants
- Advanced coatings that promote osseointegration
- Minimally invasive robotic-assisted implantation techniques
Global demand for orthopedic implants continues to grow, particularly as aging populations seek improved quality of life through surgical intervention.
Safety issues, recalls, and regulatory concerns
Despite their benefits, implantable orthopedic devices have been associated with significant safety challenges, including device failure due to wear, corrosion, or fracture; adverse biological reactions to metal ions or debris, improper fixation, leading to pain, immobility, or need for revision surgery, and component mislabeling or design flaws
Notable recalls and controversies
Biomet M2a Magnum Hip Implants. Promoted as ideal for active individuals, these metal-on-metal implants were later linked to tissue damage and metal toxicity.
DePuy ASR XL Hip System. Recalled globally after high early failure rates and metallosis in patients.
Zimmer Persona Knee Components. Voluntarily recalled due to loosening caused by poor bonding to bone cement.
These events led to growing concerns about the FDA’s 510(k) pathway, which allowed many implants to enter the market without clinical trials if deemed “substantially equivalent.” Postmarket surveillance gaps, especially when adverse events went underreported or were reported late. And inconsistent international regulatory standards, resulting in devices banned abroad still being sold in the U.S.
Looking ahead: Challenges and opportunities
As device complexity increases and expectations for personalized care rise, so too do the demands for rigorous pre- and post-market oversight. Key areas of focus include:
- Improved clinical evidence requirements prior to market clearance
- Enhanced postmarket surveillance, including real-time registries and device tracking
- Global harmonization of vigilance systems to catch safety signals sooner
- Biocompatibility testing and long-term durability studies
What patients and professionals need to know
Healthcare professionals
- Stay current with safety notices and recalls via FDA, TGA, MHRA, and other regulatory databases
- Report adverse events promptly to national authorities
- Ensure thorough informed consent processes for patients receiving implantable devices
Patients
- Ask questions about the device’s safety history
- Monitor for symptoms after implantation (pain, swelling, mobility changes)
- Keep implant information cards and report any unusual symptoms to providers
How MDP Can Help
At Medical Devices and Pharma (MDP), we provide expert support for regulatory submissions and premarket strategy, postmarket surveillance systems and audits, and gap analyses and QMSR readiness for implantable device manufacturers.
Implications of the New Federal Vaccine Schedule for Children
The New Federal Vaccine Schedule for Children: What Changed and What Are the Implications?Authors: Jennifer Kates and Josh Michaud, KFF This story was originally published by KFF.As widely expected, and following a recent Presidential memorandum, the Department of...
When Generic Drugs Are Not Equivalent
Fighting for BreathLung transplant patient Hannah Goetz’s life depended on the generic version of a critical drug. It was supposed to be equivalent to the brand-name medication — but the FDA doesn’t always ensure that’s the case.by Megan Rose and Debbie Cenziper,...
Labeling Correction for TRUE METRIX® Blood Glucose Meters
Labeling Correction for TRUE METRIX® Blood Glucose Meters Highlights the Critical Role of Clear Error Messaging Trividia Health, Inc. has initiated a labeling correction affecting all TRUE METRIX®, TRUE METRIX AIR®, TRUE METRIX GO®, and TRUE METRIX PRO® blood glucose...