Labeling serves a critical role for medical device and pharmaceutical manufacturers, providing essential information to healthcare professionals, patients, and regulatory authorities. Following are several key reasons why labeling is of utmost importance in these industries.
Patient Safety
Accurate and comprehensive labeling is vital for ensuring patient safety by providing clear instructions for the safe and effective use of medical devices and pharmaceutical products. Patients rely on labeling to understand how to properly administer medications, use medical devices, and follow treatment protocols, reducing the risk of medication errors, adverse events, and misuse.
Regulatory Compliance
Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) mandate specific labeling requirements for medical devices and pharmaceuticals to protect public health and ensure product safety and effectiveness. Compliance with regulatory labeling requirements is mandatory for market approval, product registration, and continued commercialization.
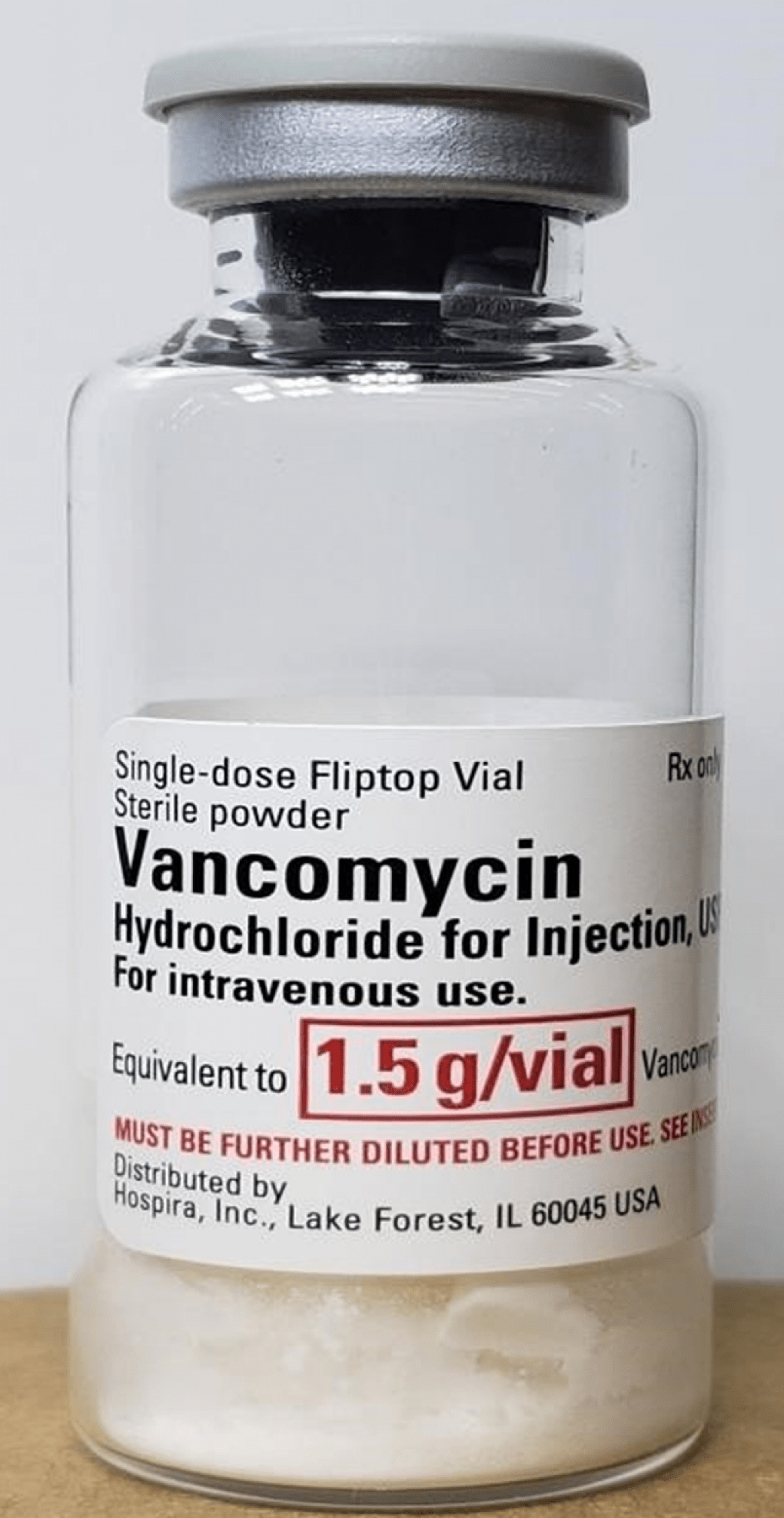
Product Identification
Labels serve as a means of product identification, enabling healthcare professionals and patients to accurately identify medical devices and pharmaceutical products, including their brand name, generic name, strength, dosage form, and manufacturer information. Proper product identification is essential for inventory management, dispensing, and administration of medications and devices.
Dosage and Administration Instructions
Labeling provides essential information on dosage, administration instructions, and precautions for medical devices and pharmaceuticals. This includes dosage strengths, frequency of administration, route of administration, special handling instructions, and contraindications. Clear and concise instructions help ensure proper use and dosage compliance, optimizing therapeutic outcomes and patient safety.
Warnings and Precautions
Labels include important warnings, precautions, and contraindications to alert healthcare professionals and patients about potential risks associated with the use of medical devices and pharmaceutical products. This information helps mitigate the risk of adverse events, interactions, allergic reactions, and other safety concerns.
Storage and Handling Instructions
Proper storage and handling are critical for maintaining the stability, potency, and integrity of medical devices and pharmaceutical products. Labels provide guidance on storage conditions (e.g., temperature, humidity), shelf life, expiration dates, and disposal instructions to prevent product degradation and ensure product efficacy and safety.
Adverse Event Reporting
Labeling often includes information on how to report adverse events or product complaints to the manufacturer or regulatory authorities. This facilitates post-market surveillance and pharmacovigilance efforts, allowing manufacturers to monitor product safety, identify emerging risks, and take appropriate corrective actions to protect public health.
Multilingual Requirements
In global markets, labeling may need to be provided in multiple languages to accommodate diverse populations and regulatory requirements. Multilingual labeling ensures that essential product information is accessible to healthcare professionals and patients worldwide, regardless of language proficiency.
Summary
In summary, labeling plays a critical role for medical device and pharmaceutical manufacturers by providing essential information on product identification, dosage and administration, warnings and precautions, storage and handling, adverse event reporting, and regulatory compliance. By ensuring accurate, clear, and comprehensive labeling, manufacturers contribute to patient safety, regulatory compliance, and effective healthcare delivery.
Contact us
Do you have questions about labeling and promotion? We are available to perform labeling and promotional reviews. Let us know – please contact us and we can provide regulatory guidance and advice for upcoming regulatory projects.