SonarMed Inc. Recalls Airway Acoustic Sensors
Restriction of inner diameter may impede catheter passageUrgent Class I Recall Notice
SonarMed Inc. has issued a Class I recall for its Airway acoustic sensors due to a critical issue that could lead to serious health consequences for patients. The U.S. Food and Drug Administration (FDA) has identified this as the most severe type of recall, indicating a risk of severe injury or even death. If you or your facility use SonarMed Airway acoustic sensors, it’s crucial to take immediate action to ensure patient safety.
Recalled Product Details
- Product Names: SonarMed Airway acoustic sensors
- Product Codes: OQU
- Model Numbers: AW-S025, AW-S030, AW-S035
- Distribution Dates: October 12, 2022, to August 11, 2023
- Devices Recalled in the U.S.: 1,800
- Date Initiated by Firm: March 21, 2024
Device Use and Function
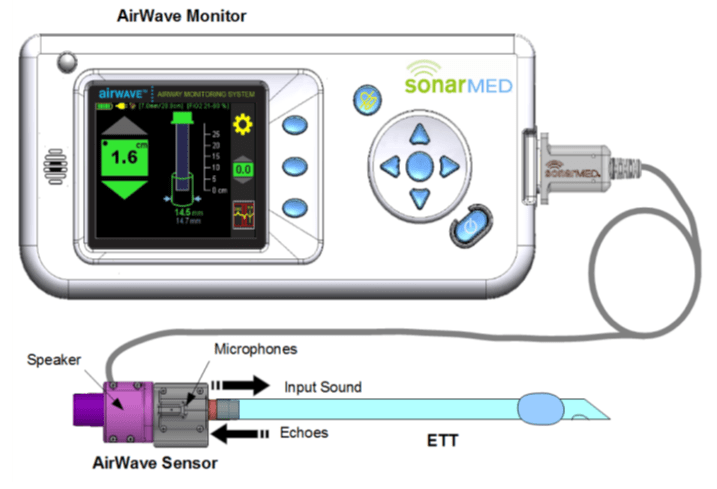
The SonarMed airway monitoring system is a critical tool used in hospital settings, including intensive care units and emergency departments. It provides real-time information to clinicians regarding the positioning and condition of endotracheal tubes (ETT) in ventilated patients. The SonarMed Sensor, a key component of the system, replaces the standard ETT connector and utilizes acoustic reflection technology for monitoring purposes.
Reason for Recall
SonarMed Inc. is recalling the Airway acoustic sensors due to a restricted inner diameter, which can impede the passage of a suction catheter. This limitation poses significant risks to patients, including delays in treatment, hypoxia, pneumothorax, hypoventilation, tissue damage, bradycardia, and respiratory failure.
Impact and Reported Incidents
While there has been one reported injury associated with the affected sensors, there have been no reports of deaths. However, the potential for serious harm underscores the urgency of this recall.
Actions for Affected Parties
If you or your facility are affected by this recall, immediate action is necessary to mitigate risks:
- Discontinue the use of affected sensors and quarantine all SonarMed Airway System products.
- Arrange for the return of quarantined sensors to Medtronic, the firm representing SonarMed Inc.
- Contact Medtronic for a Return Goods Authorization (RGA) and ensure timely return of the products.
- Complete and return the Customer Confirmation Form provided with the recall notice.
- Share this information with any parties who may have received or used the SonarMed Airway sensors.
Contact Information
For further assistance or inquiries regarding this recall, affected customers in the U.S. should reach out to Medtronic Representative or Customer Service at 800-962-9888, Option 2.
Conclusion
The safety and well-being of patients are paramount. By taking prompt action in response to this recall, healthcare providers can help prevent potential harm and ensure the continued delivery of safe and effective care. Stay informed and vigilant to protect those under your care.
Links
Read the FDA recall announcement here.