Establishing a Common Language for Quality Management Systems
ISO 9000:2015, Quality management systems — Fundamentals and vocabulary, serves as the cornerstone of the ISO 9000 family of standards, providing essential terminology and principles for quality management systems (QMS). It establishes a shared language that enables organizations across industries to communicate effectively about quality-related concepts and practices. And most importantly, in addition to 21 CFR 820 and ISO 13485, it is a reference source for FDA’s Quality Management System Requirements regulations, which go into effect on February 2, 2026.
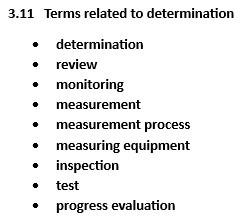
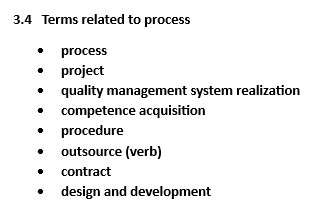
This page compiles key definitions from Clause 3 of ISO 9000:2015, offering clear explanations of terms like “process,” “product,” “quality,” and “management system.” These definitions are designed to help organizations align their efforts toward consistently meeting customer and regulatory requirements while enhancing overall efficiency and satisfaction. The definitions in the standard are divided into 13 themes, such as terms related to system, process, and organization. The themes listed in the column on the right are linked to definition lists.
Whether you are new to ISO 9000 standards or seeking a reference to deepen your understanding of its terminology, this list will serve as a valuable resource for ensuring clarity and consistency in quality management practices.
ISO 9000 Definitions
Click on an image to see an enlarged view