QMSR Integration
The integration of FDA Quality System Regulation (QSR) requirements into ISO 13485:2016 provides a unified approach for medical device manufacturers to align with global standards while meeting U.S. regulatory requirements.
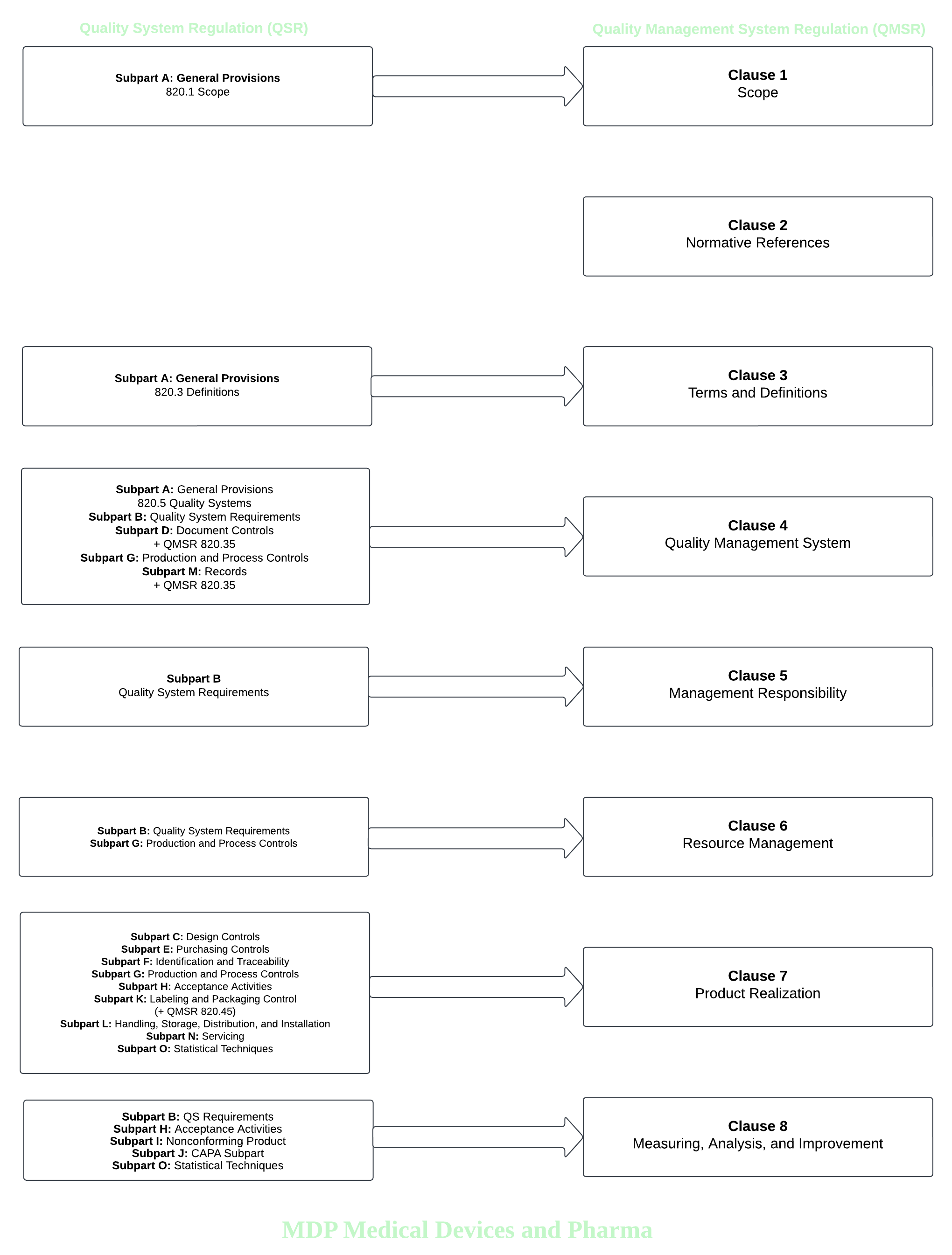
The FDA’s Quality Management System Regulation (QMSR) will shift toward harmonization with ISO 13485, streamlining compliance processes for manufacturers operating in both domestic and international markets. This transition retains key elements of the QSR while embedding them into the structured framework of ISO 13485.
By mapping these requirements, businesses can establish a comprehensive quality management system that fosters consistency, regulatory compliance, and enhanced product quality. A diagram of how the QSR will transition into the QMSR is below.